gnbt news, good on one hand bad on the other.
posted on
Oct 11, 2010 10:54AM

Edit this title from the Fast Facts Section

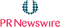
| 0.52 | +0.09 |
WORCESTER, Mass., Oct. 11 /PRNewswire-FirstCall/ -- Generex Biotechnology Corporation (NasdaqCM: GNBT) (www.generex.com) today announced that it has entered into a definitive agreement to acquire a majority interest (51%) in Global Medical Direct, LLC ("GMD") of Lenexa, Kansas, a nationwide Durable Medical Equipment and Pharmaceutical provider specializing in direct-to-consumer diabetes supplies and medications (www.globalmeddirect.com).
Generex Interim President & Chief Executive Officer, Mark Fletcher stated: "The acquisition of Global Medical Direct will be a significant step toward our goal of providing a cost effective distribution platform for our over-the-counter products and our existing pipeline of diabetes prescription products as they come to market. What's more, we can now reach diabetes patients, their doctors, and insurance companies with information about the FDA Treatment IND program for Generex Oral-lyn™, which they can take advantage of right now. In a single acquisition, we are bringing together the foundations for marketing, distribution, and education components needed to make Generex Oral-lyn™, upon FDA approval, the breakthrough product we believe it to be. I have been impressed by the success and management of GMD and believe this presents an ideal strategic opportunity for Generex."
GMD provides its growing roster of over 65,000 diabetes patients with an easy-to-use, cost effective, and convenient way for them to obtain their diabetes supplies and medications through their health plans or employer and government payors such as Medicare and Medicaid. GMD provides it patients with blood glucose meters, test strips, lancets, insulin, insulin pumps, and diabetes maintenance medications and supplies. GMD manages the reimbursement process for patients, dealing directly with insurers and payors. Generex sees great value in GMD's expertise in reimbursement issues in connection with the commercialization of Generex Oral-lyn™, Metformin gum, and the other products in its pipeline.
The closing of the transaction is subject to a number of terms and conditions, including the completion of a two-year financial audit, agreement upon ancillary agreements, favorable completion of due diligence, and attainment of sufficient financing by Generex. The acquisition is anticipated to close in early January, 2011. Terms of the acquisition will be included in a Form 8-K to be filed by Generex.
Based on a continuation of revenue and net income growth rates to date, GMD's management estimates its 2010 revenue will be approximately $30 million with net income exceeding $8 million, after successful double digit growth rates over the previous five years. GMD's 2009 revenue was over $22 million. The foregoing financial information is derived from management-prepared financial statements and has not yet been audited or reviewed by independent auditors.
In addition to providing a significant, profitable revenue stream to enhance Generex's bottom line, Generex believes the acquisition of GMD will bring a number of strategic benefits to accelerate revenue growth at Generex, including:
Approval of Reverse Stock Split
Generex management is asking its stockholders to approve a reverse stock split proposal on October 15. Management believes that a reverse stock split will put Generex in a stronger position to aggressively pursue commercialization opportunities for its buccal drug delivery and immunotherapeutic vaccine platform technologies. Based on the current price of the stock, it is anticipated that the reverse stock split ratio would not exceed 1-for-4.
If the reverse stock split is not approved, Generex's common stock will not remain listed for trading on the Nasdaq Capital Market. While the stock would then be eligible for quotation on the Over-The-Counter (OTC) Bulletin Board (OTCBB), management believes that a Nasdaq listing will afford the Company a wider variety of potentially less dilutive financing alternatives. As a development stage company, Generex must look to the capital markets to fund its on-going product development initiatives. In addition, the greater visibility and liquidity offered by a Nasdaq listing will enhance the Company's bargaining power with prospective partners and collaborators who will want comfort that Generex will have access to the capital necessary to support it contributions.
Management recognizes that many stockholders are concerned that a reverse stock split might compromise share value. However, management is not seeking the reverse stock split in a vacuum. We believe that the strength and maturity of Generex's buccal drug delivery and immunotherapeutic vaccine pipelines, together with the synergistic benefits of the proposed GMD acquisition, and financing from the proposed rights offering to stockholders, will create a solid foundation not merely for the preservation of share value but for significant growth.
Accordingly, the Board of Directors of Generex strongly urges stockholders to vote IN FAVOR of the reverse stock split proposal on October 15.
Stockholders are asked to read the details of the reverse stock split proposal set forth in the proxy solicitation materials in respect of the special meeting issued August 23, 2010 and accessible on our website at http://investor.generex.com/sec.cfm.
Should stockholders have any questions regarding the proxy voting procedures (including changing previously cast votes), please contact Legend Securities, Inc. by telephone at 877-317-7526 or via email at gnbtproxy@legendsecurities.com for US residents. Non-US residents should contact Generex directly at 800-391-6755 or contact their broker/dealer.
More on GMD:
GMD utilizes its 125+ person highly-trained, well-educated staff and call center, to maintain the ideal personal one-to-one customer relationship management environment with diabetes sufferers across the United States. Its staff establishes a continuing and ongoing dialogue with consumers to help maintain their compliance with blood glucose testing and provides them with glucometers, blood glucose test strips, lancets, insulin, and related products. GMD management expects that it will continue to recruit more than 2,500 new patients on a monthly basis. GMD is able to compile information on patient use of lancets and testing strips, thus providing a baseline for the monitoring of glucose management compliance. Glucose management compliance is of great interest to the patients' doctors and insurers and provides a reason for both groups to enroll their patients under GMD's service umbrella. Compliance in the diabetes world is essential to minimize glycemic attacks and future complications
Generex sees great value in GMD's existing diabetes educational platform. There are over 50 million pre-diabetics in the United States alone and this education strategy will allow Generex to continue to introduce and present Generex Oral-lyn™ (upon FDA approval) as well as Generex's other products to GMD's entire roster of patients as well as future consumers. Additionally, Generex, through its website and latest branding initiatives in the social media world (on Twitter, Facebook, LinkedIn, etc.), will help to serve as a platform for GMD, bringing further exposure and patient recruitment opportunities to increase its customer base.
GMD's in-house pharmacy permits the company to seamlessly handle both prescription and non-prescription drugs and handle the state-by-state intricacies of paperwork necessary for reimbursement. Generex also sees exceptional value in GMD's streamlined, and expandable packaging and shipping facility, which ships thousands of packages each month to a growing number of diabetes patients all across the United States. In anticipation of eventual marketing of Generex Oral-lyn™ when FDA approval is received, GMD's facility represents the ideal, centrally located, fully equipped national warehousing and shipping depot.
Upon completion of the acquisition, Generex intends to work closely with GMD's current management team, including Joseph Corso, Robert Shea, and Mark Franz who will remain in place to continue GMD's expansion. Joe Moscato and his team at Seahawk Capital Partners, Inc. are providing advisory services on behalf of Generex in connection with the acquisition.
Robert Shea, President & Chief Executive Officer of Global Medical Direct stated: "We are excited at the prospect of teaming up with Generex. The Generex team brings tremendous industry knowledge, expertise, and innovative technologies which will enable us to expand our market opportunities and drive both top and bottom line growth to new levels. The benefits of this combination will be immediately felt by our diabetes patients, providing them access to several new Generex OTC products, but, more importantly, they will have access to the new Generex Oral-Lyn™ buccal insulin spray product under the FDA Treatment IND program. The Generex pipeline of pharmaceutical products expected to be introduced over the next 3-5 years will position Global Medical Direct as the industry leader in the direct-to-consumer diabetes mail order segment."
About Generex Biotechnology Corporation
Generex is engaged in the research, development, and commercialization of drug delivery systems and technologies. Generex has developed a proprietary platform technology for the delivery of drugs into the human body through the oral cavity (with no deposit in the lungs). Generex's proprietary liquid formulations allow drugs typically administered by injection to be absorbed into the body by the lining of the inner mouth using Generex's proprietary RapidMist™ device. Generex's buccal insulin spray product (Generex Oral-lyn™), which has been approved in India, Lebanon, Algeria, and Ecuador for the treatment of subjects with Type-1 and Type-2 diabetes, is in Phase III clinical trials at several sites around the world. Antigen Express, Inc. is a wholly owned subsidiary of Generex. The core platform technologies of Antigen Express comprise immunotherapeutic vaccines for the treatment of malignant, infectious, allergic, and autoimmune diseases. For more information, visit the Generex website at www.generex.com or the Antigen Express website at www.antigenexpress.com. Information contained in, or accessible through, the websites of Generex or Antigen Express is not incorporated herein and is not a part of the proxy soliciting material.
Safe Harbor Statement
This release and oral statements made from time to time by Generex representatives in respect of the same subject matter may contain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These statements can be identified by introductory words such as "expects," "plans," "intends," "believes," "will," "estimates," "forecasts," "projects," or words of similar meaning, and by the fact that they do not relate strictly to historical or current facts. Forward-looking statements frequently are used in discussing potential product applications, potential collaborations, product development activities, clinical studies, regulatory submissions and approvals, and similar operating matters. Many factors may cause actual results to differ from forward-looking statements, including inaccurate assumptions and a broad variety of risks and uncertainties, some of which are known and others of which are not. Such risks and uncertainties include the risks that: (1) Generex will not obtain the stockholder approval of the reverse stock split; (2) the reverse stock split, if implemented, will fail to have the desired effect of sufficiently raising the common stock price to meet The NASDAQ Capital Market's $1.00 minimum bid price requirement for continued listing of Generex's stock; (3) the conditions to the closing of the acquisition of Global Medical Direct, LLC will not be satisfied; and (4) the anticipated benefits of the proposed right offering to stockholders will not be realized. Known risks and uncertainties also include those identified from time to time in the reports filed by Generex with the Securities and Exchange Commission, which should be considered together with any forward-looking statement. No forward-looking statement is a guarantee of future results or events, and one should avoid placing undue reliance on such statements. Generex undertakes no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. Generex cannot be sure when or if it will be permitted by regulatory agencies to undertake additional clinical trials or to commence any particular phase of clinical trials. Because of this, statements regarding the expected timing of clinical trials or ultimate regulatory approval cannot be regarded as actual predictions of when Generex will obtain regulatory approval for any "phase" of clinical trials or when it will obtain ultimate regulatory approval by a particular regulatory agency. Generex claims the protection of the safe harbor for forward-looking statements that is contained in the Private Securities Litigation Reform Act.
Generex Contacts:
Should stockholders have any questions regarding the corporate events described in this release or have questions regarding the proxy voting procedures (including changing previously cast votes), please contact Legend Securities, Inc. by telephone at 877-317-7526 or via email at gnbtproxy@legendsecurities.com for US residents. Non-US residents should contact Generex directly at 800-391-6755 or contact their broker/dealer.