
World-Class Biotech Company in the Global Nutraceutical, Vaccine and Pharmaceutical Landscape
Revolutionizing The Manner In Which Drug Formulations Are Administered


Mountain Valley MD
(CSE: MVMD) (FRA: 20MP)
https://www.mountainvalleymd.com/

Mountain Valley MD Is The Magic Gun.
MVMD Takes Existing Vaccines and Drugs - And Delivers Them Better.
Both Into The Body and By Transportation To The World.
Way Better.
For the purposes of introducing you to Mountain Valley MD (MVMD), we’ll use vaccines as an example given the state of the world since COVID-19 arrived. Specifically, we’ve all heard more about vaccines in 12 months than we have in the last 12 years. One thing we all know about vaccines is that vaccination is the safest way to protect people against infectious diseases.
BUT
One thing we don’t know or understand about vaccinations is that they are only as good as:
1. The global physical delivery system that actually gets them from the manufacturer to the hands of nurse who injects the vaccine;
2. The delivery system into your body (i.e. injection)
If either parts of these delivery systems are weak, or even fail, a vaccine loses some or even all of its potency - and that’s not good.
This is where MVMD comes in. They don’t make the vaccines, drugs or pharmaceuticals.
What they do is make their delivery better. Their physical delivery until their ultimate delivery into your body - and that is very good.
By doing so, they help save lives and they help manufacturers be more profitable - and that is very good for humanity and shareholders.
Now that you understand the value proposition of MVMD, you can begin to understand the power of MVMD’s mission statement:
Mountain Valley MD is building a world-class biotech and life sciences company organization centred around the implementation of its:
- Patented oral drug formulation and delivery technologies - Quicksome™
- Solubilization Technology : Quicksol(™) takes highly insoluble drugs and makes them solubilized (more like water) into deliverable products, making delivery more effective


WATCH THIS POWERFUL 2-MINUTE IVERMECTIN CLIP BEFORE THE US SENATE

Dr. Pierre Kory - December 8, 2020
Senate Committee on Homeland Security and Governmental Affairs
This is the delivery system into the body we talked about above and will discuss in further detail below. As you will see, it too is incredibly exciting.
The Japanese scientist who discovered Ivermectin 30 years ago, was recently awarded the Nobel Prize in Physiology and Medicine for his discovery of Ivermectin
Ivermectin is on the World Health Organization's List of Essential Medicines. It is an antiparasitic drug that was valued at US$7.2 billion in 2019 - but has suddenly become significantly more relevant as a “Wonder Drug” for dealing with COVID-19.
As you will see further below, MVMD has made the impact of Ivermectin better by several magnitudes, with 500% and 600% increases in key performance metrics, which in turn has created a massive market opportunity for the Company.
MVMD TARGET MARKETS
There are 2 markets MVMD is targeting and going after:
1. HUMAN
This is obvious and you would think it is their biggest market. It is not.
2. ANIMAL
The market for animals is not only bigger than the human market by several magnitudes, it is also an easier market to address given the fact millions of animals can be culled and hundreds of millions of dollars lost with just one virus outbreak.
MVMD 3 METHODS OF DRUG DELIVERY
1. INJECTABLE (Human & Animal)
The injectable delivery of vaccines and drugs is the most well known.
2. SUBLINGUAL (Human Only)
Sublingual, from the Latin for "under the tongue", is a way of giving medication by placing the drug beneath the tongue. They are absorbed and dissolved into the bloodstream because the area underneath the tongue has tiny blood capillaries. Therefore, drugs are absorbed directly and quickly into the body without passing through the digestive system.
The nature of sublingual delivery means it can only apply to humans.
3. TOPICAL (Human & Animal)
Topical means applying medicine to a localized part of the body and is administered by applying directly to the skin, but can also include the nose and eyes to absorb medication.
MVMD VIRUSES & DISEASES TARGETED
1. Polio
2. Malaria
3. Covid-19
4. H5N8 (Avian Flu)
5. H5N1 (Swine Flu)
6. Tuberculosis
DEEPER DIVE - THE COLD CHAIN PROBLEM, MASSIVE MARKET AND THE MOUNTAIN VALLEY MD SOLUTION
Generally speaking, a cold chain is a temperature-controlled supply chain for a product that requires a specific temperature range during distribution.
The most common example that we can all relate to is the distribution of food such as meats or milk. When we buy these at the store, they are refrigerated and have been refrigerated all the way back to when they were packaged.
They even remain refrigerated at your home until the moment you decide to consume them.
VACCINE COLD CHAINS - Most of us probably don’t realize that vaccines have to go through the same process. The government of Canada website generally describes it as follows:

The “cold chain” refers to the process used to maintain optimal temperature conditions during the
- transport
- storage and
- handling of vaccines
- The process starts at the vaccine manufacturer and ends with the administration of the vaccine to the patient.
- A high-quality cold chain allows health workers to deliver life-saving vaccines to every last child.
- Excessive heat or cold exposure can damage vaccines.
THE IMPORTANCE OF MAINTAINING THE COLD CHAIN
Vaccines are sensitive biological products that may become less effective, or even destroyed, when exposed to:
- temperatures outside their recommended range and/or
- to direct sunlight or fluorescent light.
Cold-sensitive vaccines suffer an immediate loss of potency following freezing.
Heat-sensitive vaccines will suffer some loss of potency following temperatures above their range. Repetitive exposure to higher temperatures results in loss of potency.
Over 95% of all approved biologics and 90% of all vaccines are cold chain dependent. According to the data presented by the WHO, the current global vaccination coverage is nearly 85%. However, it has been reported that 25% of vaccines are damaged due to cold chain malfunction. In some countries, about 80% of the drugs are estimated to lose their potency due to inadequate temperature control during their cold chain transportation.
WHAT CAUSES THE COLD CHAIN TO BREAK?
There are many situations that can cause a vaccine cold chain to break including:
- equipment failures
- power outages
- natural disasters
However, the biggest cause of a vaccine cold chain break comes from the fact that people who need vaccines the most - the most vulnerable - live in areas with poor infrastructure, let alone refrigeration.

According to the NCBI:
Vaccine preventable diseases like polio, measles, and hepatitis are a major cause of morbidity and mortality among children in developing countries. Vaccination is one of the most effective disease prevention strategies when implemented properly across all sections of the at-risk population. Immunization against a disease is achieved only if a potent vaccine is administered. The system used for keeping and distributing vaccines in good condition is called the “cold chain.”
For some vaccines, even the slightest variations in temperature / storage conditions can adversely alter product integrity and / or viability.
Unfortunately, vaccination rates in India indicate a considerable disparity between children in urban compared with rural areas. Only 27% of the population lives in the urban parts of the country. The weak health infrastructure and unsanitary conditions contribute to the increased incidence of diseases like polio, cholera, and hepatitis in rural areas compared with the urban areas.
MVMD QUICKSOME(™) ELIMINATES THE COLD CHAIN
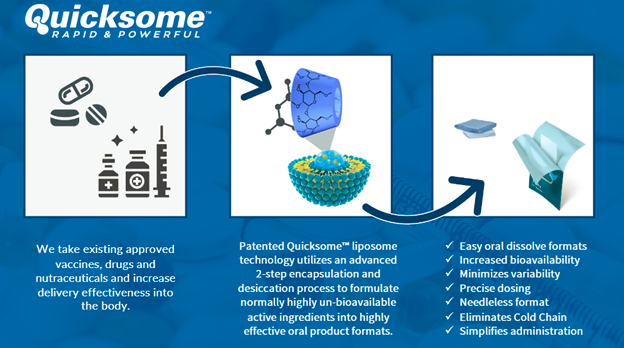
On January 14, 2021 Mountain Valley MD Was Awarded A Canadian Patent for its Quicksome™ Technology. Quicksome™ uses an advanced two-step process to formulate active ingredients, which previously could not be absorbed and used by the body, into highly effective oral product formats.
Quicksome™ technology is being applied across a variety of vaccine, drug and nutraceutical applications across numerous continents:

PRE-CLINICAL DATA SHOWS IVERMECTIN ABSORPTION VASTLY IMPROVED BY QUICKSOME™
On December 10, 2020, MVMD released pre-clinical trial data where a solubilized version of the drug Ivermectin was administered via its Quicksome™ technology, as compared to an existing oral Ivermectin solution. The results demonstrated the Quicksome™ technology was able to successfully administer Ivermectin across the mucosa (The moist, inner lining of some organs and body cavities ), with a significant improvement in the pharmacokinetic performance (the movement of drug into, through, and out of the body) compared to current oral formats.
HOW MUCH BETTER WAS IVERMECTIN WITH Quicksome™?
The pre-clinical data included:
- A 500% increase in absorption and use by the body compared to oral tablets;
- A 600% increase in TMAX of just 1 hour (the time to reach the maximum concentration in the body) compared to oral tablets;
- 0% decline in CMAX (peak concentration that a drug achieves) over the entire 6-hour period investigated
- Only 5% variability compared to 40% variability for oral tablets.
Just how substantial are these improvements? This quote says it all:
“MVMD Has Succeeded In Making A Nobel Prize Winning Wonder Drug Even Better Based On Overcoming Its Number One Limitation Of Solubility.”
Mike Farber, Chief Science Officer at Mountain Valley MD

“For me personally, the idea that our delivery technology could help dispense drugs such as insulin or deliver vaccines into the body through a rapid dissolve sublingual strip and eliminate the need for painful needle injections is truly game changing.”
Dennis Hancock, President and CEO of Mountain Valley MD


DEEPER DIVE - IVERMECTIN THE “WONDER DRUG”, MARKET SIZE, PROBLEMS AND THE MOUNTAIN VALLEY MD SOLUTION

MALARIA, POLICY & GLOBAL DEVELOPMENT
INTRO
Quicksol(™) takes highly insoluble drugs and makes them solubilized (more like water) into deliverable products.
WHY IS SOLUBILITY SO CRITICAL
Poor solubility causes low bioavailability (the degree and rate at which a drug is absorbed into the body). As you can imagine, low solubility and absorption often decrease a drug’s efficacy. As a result, poorly soluble drugs require higher doses in order to reach their optimal therapeutic levels, leading to problems such as gastrointestinal mucosal toxicity (a membrane that lines various cavities in the body and covers the surface of internal organs) - and you don’t need a medical degree to know that isn’t good.
On its face, this seems both logical and probably simple to solve. It isn’t. In fact it’s a major problem for both society and the businesses of pharmaceutical companies.
HOW BIG OF A PROBLEM IS SOLUBILITY? MEDICATIONS MAY NEVER MAKE IT TO PATIENTS WHILE WASTING DRUG DEVELOPERS TIME AND MONEY
“It is generally recognized that poor solubility is one of the most frequently encountered difficulties in the field of pharmaceutics.”
Kumar and Singh. American Journal of Pharmacological Sciences
Poor solubility can lower a drug’s chance of marking it to market.

As you can see, this is a problem because drug developers waste time and money on medications that will never make it to patients.
ENTER QUICKSOL(™)
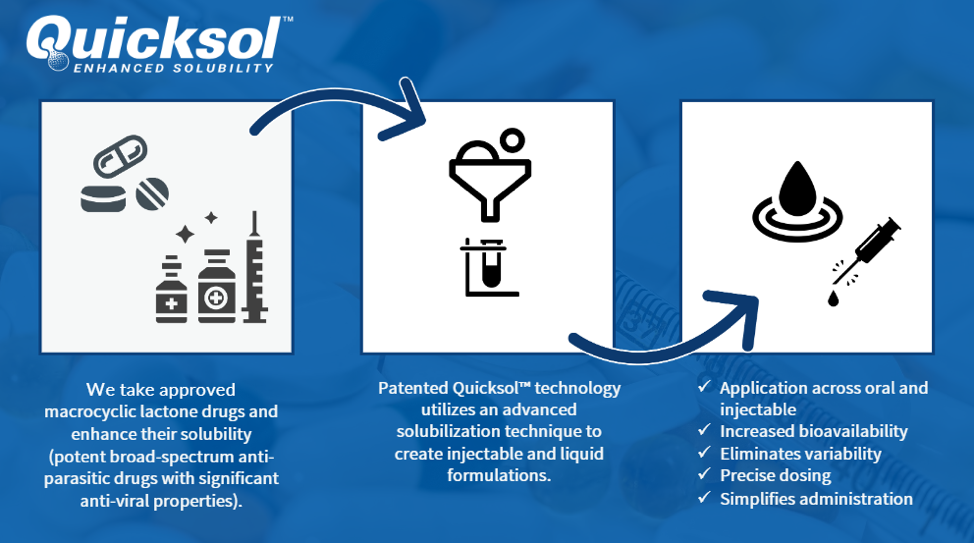
Quicksol™ WITH IVERMECTIN IMPROVES SOLUBILITY AND DRUG DELIVERY
Enabling enhanced injection or other liquid application of poorly soluble drug to enhance bioavailability with new delivery options.
Simply put, Quicksol takes known drugs that are inefficient in delivering their intended medicinal purpose and makes them soluble. Quicksol™ improves them by increasing the ability to deliver more of the drug effectively through increased solubility
- Applications are both Oral and Injectable
- Increases Bioavailability, which is the means to how much is delivered
- Eliminates variability Why is this important
- Provides precise dosing
QUICKSOL TECHNOLOGY PROJECTS INVOLVING IVERMECTIN
The use of Ivermectin in Canines is being studied in 2021 and early results demonstrate that an injection into the muscle increases bioavailability by 800% versus injecting subcutaneously, (which is the layer between the skin and muscle)
- Early results show the effectiveness in 15 minutes versus normal 36 hours for injection Ivermectin has been accepted for COVID-19 therapeutic clearance:
On January 14 2021: The US National Institute of Health (NIH - US Department Of Health & Human Services) Revised It's Treatment Guidelines for Ivermectin for the Treatment of COVID-19
Research & Trial Updates September 2021
ONCOLOGY
- As announced on May 3, 2021, pre-clinical trials for triple-negative breast cancer, metastatic melanoma and Lewis Lung Carcinoma were conducted over the past several months. The results of the research conducted presented some noteworthy exploratory findings that have resulted in MVMD expanding its oncology work across these tumor types with further research studies being executed.
- The Company is continuing to expand its relationships with experts in clinical and research focused oncology to pursue advanced understanding of Quicksol™️ technology applications across a broader array of insoluble molecules that have documented anti-cancer effects from prior independent research.
HUSBANDRY ANIMALS
- The Company has completed the scheduled husbandry animal trials in Bangladesh, previously announced on March 16, 2021, that were conducted with MVMD’s injectable solubilized Ivermectin technology, Ivectosol™️ 1%.
- The studies were conducted under the supervision of The People's Republic of Bangladesh’s Ministry of Fisheries & Livestock and have informed the requirements for final commercialization pathway. This will include working to complete and provide Ivectosol™️ 1% extended stability data and completion in parallel of dosing and formulation testing across a broad spectrum of animal species.
- The Company has commenced its commercialization planning with local partners inside Bangladesh in anticipation of all necessary government approvals for full Ivectosol™️ 1% manufacturing and product distribution in 2022.
FARMED FISH
- The Company has been working with The People's Republic of Bangladesh’s Ministry of Fisheries & Livestock on a unique project to evaluate the ability to administer its Ivectosol™ 1% formula in a farmed fish environment to explore its potential to impact positive health outcomes.
- MVMD invented a novel fish food application that enables Ivectosol™ 1% to be delivered to fish in an aqueous environment, with the objective of helping to prevent parasitic outbreaks that cause mortality and limit healthy growth of fish.
IVECTOL™
- MVMD has garnered interest in its work with the ivermectin drug molecule and has had determined that there is immediate demand for generic ivermectin while the Company develops out its solubilized Ivectosol™ 1% applications.
- To satisfy current business development discussions and requests for large scale production of ivermectin tablets, MVMD has coordinated pharmaceutical production of its own branded ivermectin product called Ivectol™, which is packaged in a 20-tablet box containing Ivermectin USP 12 mg tablets.
- Ivectol™ is finalizing registrations in key target markets to allow for export, as well as import and sale in approved countries.
Mountain Valley MD is building a world class biotechnology company addressing the delivery issues faced by the vaccine industry, taking existing vaccines and drugs, and delivering them better.
Received its third-party Bio Safety Level 4 (“BSL-4”) lab study results from its recent COVID-19 viral clearance study conducted with its solubilized Ivermectin technology - Ivectosol™.
Study Results
- A single dose of 2.5 milligrams per kilogram of Ivectosol™ was effective at interfering with viral replication and driving viral clearance of the B.1.1.7 COVID-19 variant.
- Tests done in vitro showed the same antiviral effect at 5uM Ivectosol™ concentration after 24 hours and again after 48 hours against all three COVID-19 variants tested - the original B.1.1.7 variant, the South African B.1.351 variant, and the P.1 Brazil variant.
